Fragile X Syndrome (FXS) is the most common form of hereditary intellectual disability and has been linked to various neurologic and psychiatric conditions. The FMR1 gene product, FMRP, controls mRNA metabolism in the brain and consequently regulates the production of crucial molecules involved in receptor signaling and spine development. FXS is brought on by a triplet expansion that prevents the FMR1 gene from being expressed. Speech delay, sensory hyperarousal, and anxiety are the most typical FXS symptoms in children. Although there is currently no known cure for FXS, the new peptide technology has paved the way for therapy that could help offer a novel and promising approach to treating FXS.
What is Fragile X Syndrome?
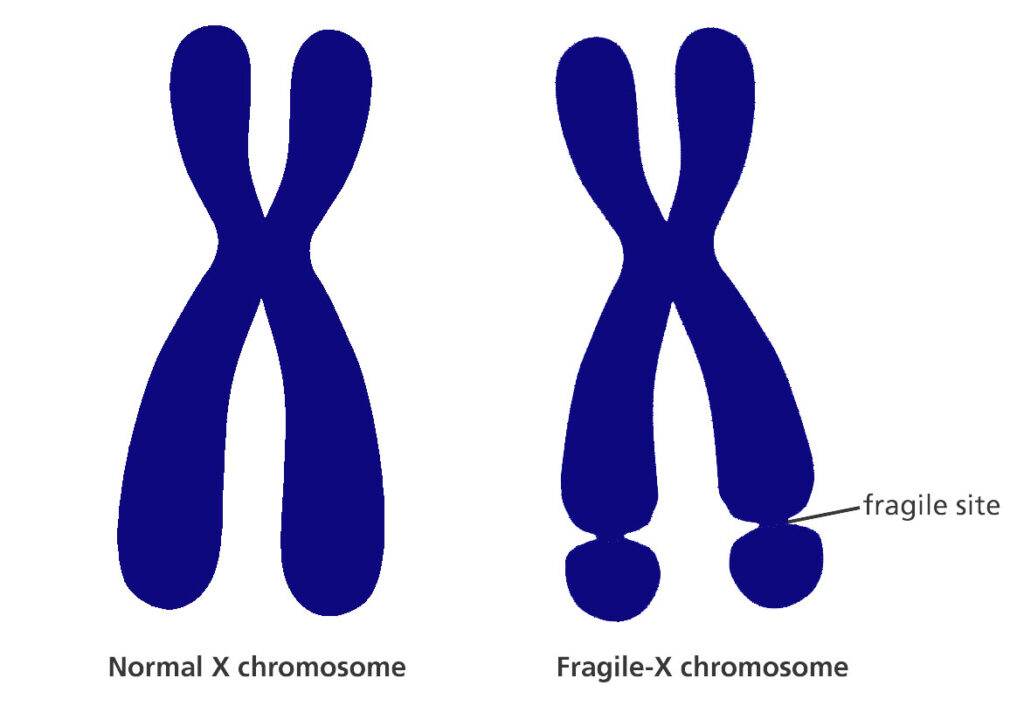
Fragile X syndrome (FXS) is a genetic condition that causes mild-to-moderate cognitive disability. This disease is commonly caused by an extension of the CGG triplet repeat inside the FMR1 (fragile X messenger ribonucleoprotein 1) gene on the X chromosome. This has the effect of silencing (methylating) this part of the gene and depleting the FMRP protein, which is necessary for the healthy growth of neuronal connections.
People with FXS present with intellectual disabilities, autism, hyperactivity, a long or narrow face, huge ears, and macroorchidism (abnormally large testes) during puberty. The most common symptoms of FXS in young children are speech delay, sensory hyperarousal, and anxiety. Compared to boys, girls are less afflicted with intellectual disability–roughly two-thirds of affected females have intellectual disabilities while the average IQ of FXS-affected males is below 55.
What are the Symptoms of Fragile X Syndrome?
Males with a full mutation often have a penetrance of approximately 100% and nearly invariably show signs of FXS, but females with a full mutation typically have a penetrance of around 50% due to the presence of a second, normal X chromosome. Symptoms in females with FXS can range from mild to severe, however, they are often less severe than in males.
Even so, most young children display no physical signs of FXS since the physical features of FXS typically appear in puberty. Aside from intellectual disability, noticeable features of the condition may include an elongated face, huge ears, flat feet, large testes (macroorchidism), and low muscle tone.
These are some of the symptoms of Fragile X Syndrome:
- Individuals with fragile X syndrome often have learning difficulties (IQ less than 70) and delayed milestones, as well as common physical features such as a long or narrow face, big testicles (2-3 times the average size), facial asymmetry, a prominent jaw, and huge ears.
- Other symptoms may include hand flapping, repetitive movements, clumsiness, avoiding eye contact, seizures, and disturbed sleep.
- Specific speech problems may include echolalia and perseveration (the inability to complete a sentence due to repetition of words at the end of a phrase).
- There could be associated anxiety-related symptoms such as obsessive-compulsive and perseverative behavior patterns, emotional instability, and aggressive or self-aggressive behaviors. Shyness and social disengagement issues are more common among affected females.
- In certain situations, those affected may also be diagnosed with autistic spectrum disorder or attention deficit hyperactivity disorder (ADHD). About 30% of the afflicted men have autism, and many of them may also have an autistic spectrum condition.
What Causes Fragile X Syndrome?
Fragile X syndrome is caused by a mutation in the FMR1 (Fragile X Messenger Ribonucleoprotein 1) gene, which stops the body from producing a crucial protein called FMRP. This protein contributes to producing and maintaining connections between brain cells and the nervous system. When FMRP is missing, impulses from the brain may be misdirected. This results in the developmental and learning issues seen in Fragile X Syndrome.
Males and females are both impacted by FXS. However, compared to men, women frequently have milder symptoms. This is due to the location of the FMR1 gene which is on the X chromosome, and females (who have two X chromosomes) have two copies whereas males (who have one X chromosome) have just one. There is no backup if this one X chromosome fails.
How is Fragile X Syndrome Diagnosed?
Early diagnosis
According to studies, FXS is frequently diagnosed in young children under the age of 3 with speech delay. Boys with FXS frequently have attention deficit hyperactivity disorder (ADHD), which is characterized by considerable impulsivity and anxiety, as well as repeated speech patterns, hand biting, hand stereotypes, rocking, and even head banging. These behaviors, along with social and linguistic difficulties, often lead to a diagnosis of autism spectrum disorder (ASD) before the diagnosis of FXS is made. Approximately 30% of boys with FXS match the diagnostic criteria for autism, and these kids have the lowest developmental and adaptive behavior scores among those with FXS.
Clinical observations
A family history of ID, ASD, neurological issues (such as tremors, ataxia, or dementia in one of the grandparents), or early menopause (before 40 years of age) may prompt the diagnosis of FXS in the family.
The first stage of examinations can be done by testing DNA using a blood sample or chorionic villus tissue. The majority of labs combine southern blotting (which detects complete mutations) with PCR testing (identifies pre-mutations and smaller CGG repeats). During southern blotting, DNA material is transferred from an agar gel to a membrane. This membrane can be subjected to electrophoresis to determine a specific DNA sequence. In order to amplify a specific DNA region and facilitate identification, PCR is used as a polymerase enzyme.
The second stage of examination includes CGG methylation testing to assess FMRP synthesis (‘silencing’) and molecular approaches to detect loss of function mutations. The development of long-range-PCR-based techniques using DNA from either chorionic villi or amniocytes has enabled antenatal testing.
Late diagnosis
Research shows, a late diagnosis may occur in older patients who had genetic testing prior to the 1991 discovery of the FMR1 gene, or in patients with a mild form of the disease with unusual symptoms, as is common with the UFM alleles. There have been instances where people with FXS have been institutionalized during their adolescence or adulthood without undergoing a diagnostic examination to determine the cause of their intellectual disability. Furthermore, the majority of women who have FM typically don’t have an intellectual disability, and they commonly get the FM diagnosis after their children are diagnosed with FXS.
How to Treat Fragile X Syndrome?
Several studies have been conducted in an attempt to find specific treatments for FXS that can alleviate some of the symptoms and improve the life quality of the affected patient. Some of the medications used include mechanisms of action that focus on epigenetic modification, the glutamatergic system, and the control of the translation of FMRP target mRNAs.
Over the past ten years, significant progress has been achieved in the research and development of novel pharmaceutical treatments for FXS. At the same time, peptide therapeutics have been developed to treat a number of diseases.
Researchers believe that from this viewpoint, these two paths may eventually cross, bringing a new era of pharmacotherapeutic treatment for FXS patients. Chemical agents that inhibit various pathways unregulated in FXS are now the most studied approaches for FXS treatment. However, the cause of FXS is a genetic abnormality (FMR1 silencing), and effectively correcting the loss of FMRP protein is still a challenge.
The new peptide technology, in conjunction with more improved computational approaches and a number of protein structures published in databases, presents an alternative and powerful method for developing a more selective and safe medication targeting those protein complexes that may be regarded as the “main actors” in the FXS pathology.
The key benefits of using peptides over other pharmaceutical molecules are selectivity, tolerability, predictable metabolism, the ability to target PPIs (protein-protein interactions), and decreased synthesis complexity. Furthermore, peptide treatment would not be a long-term therapy; rather, these molecules would be supplied just during the first years of the life of FXS children, when the brain is still remodeling, to allow correct synaptic network creation. In light of this, researchers argue that peptides could help compensate for the FMRP shortage by restoring the balance between protein synthesis and actin dynamics, offering a novel and promising approach to treating FXS.